Article Text
Statistics from Altmetric.com
Neurology, perhaps more than any other field of medicine, relies on a close association between clinical phenotype and underlying pathology. This is certainly the case for the neurodegenerative diseases where despite advances in genetics and biomarkers a careful clinical assessment remains the key to accurate diagnosis. In this context, Alois Alzheimer's description of the association between progressive cognitive decline centred on episodic memory and the presence of amyloid plaque and tau tangle pathology remains central to our formulation of Alzheimer's disease—although his original case was highly atypical, being a young woman now known to have an autosomal dominantly inherited form of the disease.1
A fundamental question that underlies all neurodegenerative disorders is why certain pathologies associate fairly reliably with certain clinical phenotypes. Seminal pathology studies in Alzheimer's disease showed that amyloid plaque and tangle pathology spreads through the brain in a fairly predictable sequence, starting in the medial temporal lobe before involving other neocortical structures;2 this sequential spread—particularly of tau pathology—correlates broadly with the typical progression of symptoms. More recently, the availability of biomarkers has allowed for other aspects of the pathological cascade to be assessed in vivo. Cerebrospinal fluid allows for measurement of brain-enriched proteins, inflammation and synaptic dysfunction; positron-emission tomography allows for visualisation of abnormal deposits of amyloid and tau, and patterns of glucose metabolism; and MRI techniques allow for quantification of neuronal cell loss (atrophy), and for interrogation of structural and functional connectivity, perfusion and tissue microstructure. Together, these techniques are opening up ever earlier and more accurate diagnosis,3 as well as providing insights into the interaction between different pathological processes. Thus, the pathology of Alzheimer's disease is emerging as a much more dynamic process than previously thought, likely to include accumulation of—and interaction between—several different protein moieties, inflammation, self-promoting propagation of pathology through a vulnerable ‘default mode’ network, perhaps via prion-like spread, synaptic breakdown, neurochemical loss and neuronal cell death, all of which occur well before the emergence of symptoms.
While amyloid plaques and neurofibrillary tangles are most commonly associated with an amnestic syndrome, individual patients inevitably have different constellations and degrees of cognitive symptomatology, with some having sufficiently unusual phenotypes to be considered as having distinct disease variants.4 These include patients with prominent dysexecutive or behavioural problems (frontal Alzheimer's disease), those presenting with word-finding difficulties and pauses in speech (logopenic aphasia), with perhaps the most striking clinical phenotype being those with various combinations of cortical visual dysfunction, apraxia and dyscalculia with relative sparing of episodic memory (posterior cortical atrophy). Beh et al5 provide a valuable clinical overview of the posterior cortical atrophy syndrome, with a particular focus on the often very striking and distinctive visual phenomenology these patients experience.
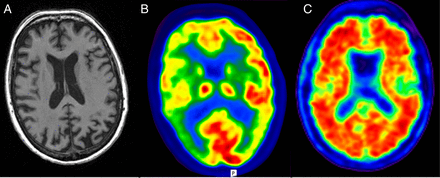
How can the same broad underlying pathology be associated with such markedly different clinical manifestations? Aside from having very different initial symptoms, there are several other notable differences between the posterior cortical atrophy and amnestic forms of Alzheimer's disease.6 Patients with posterior cortical atrophy are usually (although not exclusively) younger, typically with disease onset in the 50s or 60s. While pathological studies show that the extent and topography of amyloid deposition it is not absolutely identical in amnestic and posterior cortical atrophy-Alzheimer's disease, amyloid burden as measured using positron-emission tomography shows global cortical deposition in both variants, in marked contrast to the posterior neuronal loss and hypometabolism typical of posterior cortical atrophy (figure 1). There is also evidence for important similarities and key differences in the pattern of network breakdown in the different Alzheimer's disease variants.7 Finally, there may be genetic differences between posterior cortical atrophy and amnestic Alzheimer's disease, with hints that despite their typically young age of onset, patients with posterior cortical atrophy may be less likely than expected to carry the most common genetic risk for sporadic Alzheimer's disease, an ApoE E4 allele.8
Structural, functional and molecular imaging in posterior cortical atrophy due to Alzheimer's disease. Volumetric T1 MR brain imaging (A) shows prominent posterior volume loss, 18F-fluorodeoxyglucose positron-emission tomography (PET) scanning (B) shows cortical hypometabolism (cool colours) most prominent in both parietal lobes, while 18F-florbetapir PET (C) shows widespread cortical amyloid deposition suggesting that phenotypical diversity in Alzheimer's disease cannot all be explained by regional amyloid deposition. (Note: For clinical purposes, 18F-florbetapir images should be interpreted on a grey rather than colour scale).
Identifying common and discordant genetic (and environmental) risk factors for posterior cortical atrophy and typical Alzheimer's disease, combined with neuroimaging, cerebrospinal fluid and other biomarkers, may provide fundamental insights into Alzheimer's disease pathogenesis. It is entirely plausible that different risk factors influence the rate, timing and site of amyloid deposition; whether or when amyloid deposition leads to neurodegeneration; and which neuronal networks bear the brunt of the disease, in turn influencing how pathology spreads through the brain, and what symptoms predominate. Scientific interest aside, neurologists should be alert to the fact that Alzheimer's disease can present with unusual phenotypes, and that these are important to recognise to allow prompt diagnosis and appropriate treatment, support and guidance (eg, for posterior cortical atrophy, see http://www.ucl.ac.uk/drc/pcasupport). It is equally important not to overlook the existence or emergence of impairments in visual and other non-memory functions even in patients presenting with amnestic Alzheimer's disease, noting that such deficits were found even in Alois Alzheimer's index case, whom he noted: ‘…holds the book in such a way that one has the impression that she has a loss in the right visual field’.9
Acknowledgments
This work was supported by the NIHR Queen Square Dementia Biomedical Research Unit. The Dementia Research Centre is supported by Alzheimer’s Research UK, Brain Research Trust and The Wolfson Foundation. The images in the figure were acquired as part of a study funded by AVID Radiopharmaceuticals (a wholly owned subsidiary of Eli Lilly) and the UK FTD support group.
Footnotes
-
Contributors JMS wrote the initial draft and CFS and SJC redrafted it. All authors agreed on the submitted version.
-
Competing interests None.
-
Ethics approval NRES Committee London—Central.
-
Provenance and peer review Commissioned. Internally peer reviewed
Linked Articles
- Editors' commentary
- Review
Other content recommended for you
- Biomarkers in dementia: clinical utility and new directions
- Hiding in plain sight: a closer look at posterior cortical atrophy
- Selective vulnerability in neurodegeneration: insights from clinical variants of Alzheimer's disease
- Amyloid PET imaging in clinical practice
- Greater medial temporal hypometabolism and lower cortical amyloid burden in ApoE4-positive AD patients
- Alzheimer's disease: mimics and chameleons
- The language profile of posterior cortical atrophy
- Dementia
- Down syndrome with posterior cortical atrophy
- Cerebrospinal fluid biomarkers in the differential diagnosis of Alzheimer's disease from other cortical dementias