Article Text
Abstract
Ketogenic dietary therapies are an effective treatment option for children with drug-resistant epilepsy. There is an increasing worldwide interest in using these diets to manage adult epilepsy; uncontrolled studies show similar response rates to those in children. Despite this, there are only a few centres with dedicated services for adults. We clearly need controlled studies of this treatment in adults. Here, we aim to familiarise adult neurologists with the evidence base for these diets and give practical advice on starting and maintaining them in adults.
- EPILEPSY
- seizures
- ketogenic diet
- older people
Statistics from Altmetric.com
Introduction
There has been an increasing interest worldwide in using ketogenic dietary therapies to manage adults with drug-resistant epilepsy. Despite this, these diets are still predominantly used in children: approximately 15 National Health Service hospitals across the UK offer ketogenic dietary therapies to children with drug-resistant epilepsy, but there are only three centres for adults (Matthew's Friends clinics for Ketogenic Dietary Therapies, The National Hospital for Neurology and Neurosurgery, and Barberry, Birmingham and Solihull Mental Health Foundation Trust). The literature on these diets is also predominantly in children and we clearly need controlled studies in adults. The major barrier to treating adults with these diets appears to be the ‘lack of training, comfort, or familiarity with dietary treatments’ among clinicians caring for adults with epilepsy.1
The history of ketogenic dietary therapies
Following reports of the effects of fasting on seizure cessation, Wilder2 aimed to mimic the state of starvation by producing ketosis using a high-fat, low-carbohydrate diet. This led to the so-called ‘classical ketogenic diet’, typically with a 4:1 ratio of fat (in grams) to protein and carbohydrate combined (in grams), for people with drug-resistant epilepsy. It is possible to use lower ratios (eg, 3:1 or 2:1), depending on individual tolerability, levels of ketosis and protein requirements. The first study in 1930 included 100 adolescents (aged<16 years) and adults, treated with classical ketogenic diet monotherapy for 1 year: 12% became seizure-free, 44% ‘improved’ and 44% showed no change in seizures.3
The discovery of diphenylhydantoin in 1938 dampened the initial enthusiasm for these diets, as did the advent of new, easy-to-administer antiepileptic medications. However, although these medications were widely used, there were soon concerns regarding their adverse effects, such as gingival hyperplasia.
In the 1970s, in an attempt to make the diet more palatable, Huttenlocher et al.4 introduced the medium-chain triglyceride ketogenic diet on the premise that these triglycerides are more ketogenic per calorie; the diet therefore allowed a greater bulk of protein and carbohydrate. This diet originally derived 60% of its calories from medium-chain triglyceride oil. A modified form of the diet, designed to limit gastrointestinal side effects, derived 30% of its calories from medium-chain triglyceride oil and 30% from long-chain fats.5 Nowadays, the proportion of energy from medium-chain triglyceride fats depends on individual requirements and tolerance.
Since the early 2000s, the repertoire of ketogenic dietary therapies has expanded to include more ‘relaxed’ variant forms, including the modified Atkins diet (now more commonly as known as modified ketogenic therapy in the UK) and the low glycaemic index treatment, aiming to provide increased flexibility and palatability. The modified Atkins diet is based on a ratio of approximately 1:1, although this is not necessary in all meals, and includes 10–30 g of carbohydrate per day with no restriction of fluids, calories or protein. It allows users more flexibility and does not require the weighing of food portions or an initial hospital stay.6 Low glycaemic index treatment includes a higher proportion of carbohydrates (40–60 g/day) than the classical ketogenic diet, with 60% of calories taken from fat, but permits only carbohydrates with a glycaemic index of <50 relative to glucose.
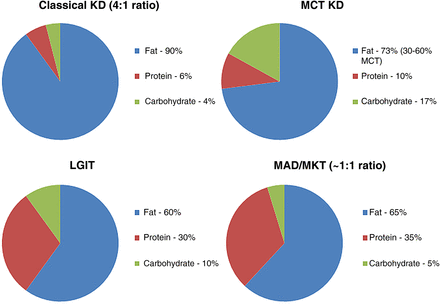
Figure 1 summarises the composition of various ketogenic diet therapies. The dietician usually fine-tunes these diets.
Composition of ketogenic diets and variants. KD, ketogenic diet; LGIT, low glycaemic index treatment; MAD, modified Atkins diets; MCT, medium-chain triglyceride; MKT, modified ketogenic therapy.
Effectiveness of ketogenic dietary therapies
Systematic reviews of ketogenic dietary therapies in children with epilepsy (although some studies included participants aged >18 years) suggest that 33%–56% of children achieve ≥50% seizure reduction and 16% achieve seizure freedom.7 ,8 In a meta-analysis, the pooled OR for achieving >50% seizure reduction among children who followed the diet until the end point of the study (this varied between studies) relative to those who had stopped the diet before the end point was 2.25;9 52% of those who stayed on the diet for up to 24 months achieved ≥90% seizure control and 24% achieved complete seizure control. These reviews and meta-analysis collated evidence only from uncontrolled and predominantly retrospective studies.
The first randomised controlled trial that assessed the effectiveness of ketogenic dietary therapies for drug-resistant epilepsy included children aged 2–16 years with either at least daily seizures or more than seven seizures per week, who had not responded to at least two antiepileptic medications.10 Thirty-eight per cent of those treated with a classical ketogenic diet or medium-chain triglyceride ketogenic diet achieved >50% seizure reduction at 3 months, compared with 6% of controls; 7% of treated participants experienced >90% seizure reduction compared with zero controls. There was no significant difference in the number of children achieving >50% or 90% reduction between the classical ketogenic diet and medium-chain triglyceride ketogenic diet groups.11 The likely reason for the responder rates being lower than those previously reported in uncontrolled studies was the trial's intention-to-treat analysis.
There was also a randomised controlled trial of the modified Atkins diet in children with epilepsy.12 52% of 50 children on the diet achieved >50% seizure reduction at 3 months, compared with 12% of 52 controls; 30% of the treatment group achieved >90% seizure reduction, compared with 8% of controls.
Despite these promising response rates in children, there have been few studies of ketogenic diet therapies in adults, presumably due to the assumption that they would find it difficult to comply with such dietary restrictions and due to fear about potential side effects. Reviews13 ,14 found 10–13 publications, including <300 adolescents and adults with epilepsy following ketogenic dietary therapies, all of which were open label and uncontrolled, with the exception of 10 adolescents (aged 12–16 years) included in the UK randomised controlled trial.10 ,11 In comparison, there are data from >1300 children treated with ketogenic diet therapies.14 Probably 30%–40% of adults achieve ≥50% seizure reduction with these diets, although fewer than 10% achieve 90% seizure reduction or seizure freedom;13–15 this is comparable with the effectiveness rates in children.
Both in children and adults, there is conflicting evidence regarding which epilepsy or which seizure types respond better to these dietary therapies, or which type of diet or ratio is more effective: a recent review found no strong evidence that any specific factors affect response.16
However, the benefits of ketogenic dietary therapies can go beyond seizure reduction alone. Adults may report improved psychological function, alertness, concentration and general quality of life when taking the diet.17–20
Tolerability
Like most medical treatments, these diets may cause adverse effects. In a meta-analysis in children, the most frequently reported adverse effects were constipation (14% of children remaining on the diet for at least 3 months); weight loss, growth problems or anorexia (13%); nausea and vomiting (5%); behavioural problems or irritability (4%); increased serum cholesterol or triglycerides (4%); lethargy (4%); hypercalciuria (2.5%); increased liver enzymes (2.4%); renal stones (1.9%); diarrhoea (1.6%) and hypoglycaemia (1.3%).9
In the randomised controlled trial of the classical ketogenic diet, medium-chain triglyceride ketogenic diet10 and the modified Atkins diet trial,12 the most common adverse effect was constipation during the first 3 months of treatment (33% and 46% of participants respectively in the two trials).
There has been no direct comparison between adolescents/adults and children of tolerability of these diets. From the limited studies available, adverse effects appear similar in all age groups; those reported in older patients tend to be non-critical and/or transient and do not lead to stopping treatment.14 One common concern in adults is hyperlipidaemia, but this returns to normal after stopping the diet.19 ,21 In a recent study, total serum cholesterol and low-density lipoprotein concentrations increased over the first 3 months in adults following the modified Atkins diet, but returned to normal within a year of treatment, even in those taking the diet for >3 years.22 Weight loss is common in adults13 ,14 although this may provide further incentive for people who are overweight or obese. Two studies found that the diets interfered with social interaction in adolescents,23 ,24 although none found that parental stress increased.14 Despite reports of loss of bone mass25 or mineral content26 in children, a case series found no negative effects on bone mineral content or density in three adults with glucose transporter 1 deficiency syndrome who had followed the diets for >5 years.27
Compliance and retention
Two studies noted adherence to the diets, measured in children by communication with them and/or their parents28 or, in adults, using serum β-hydroxybutyrate or urine acetoacetate concentrations.21 However, ketone body production and maintenance varies greatly between individuals, and the response to the diet may not depend solely on ketosis per se. Continual recording of food intake, as in one case,29 may provide more accurate levels of adherence to the diet but would not be feasible on a wider scale or in outpatient settings. We clearly need alternative ways to assess this.
A recent meta-analysis of adherence in adults reported combined rates of 45% for all types of ketogenic diet therapies, 38% for the classical ketogenic diet and 56% for the modified Atkins diet.15 Adults were significantly more likely to adhere to the modified Atkins diet than to the classical ketogenic diet. However, adherence rates appeared to be extracted from the number of patients said to be following treatment at the end point of each study, with no specific measure of the extent to which patients were actually adhering to the diet.
The available studies show widely variable retention rates. The dropout from the classical ketogenic diet ranges between 10% and 88% compared with 0% and 63% with the modified Atkins diet;14 in another review, overall 51% of ketogenic diet-treated adults and 42% of modified Atkins diet-treated adults stopped the diet before study completion.13 Many adults may refuse to consider these diets: in one study, only 18 out of 130 eligible adults consented to start them.30
The place of ketogenic dietary therapies in the overall treatment of epilepsy
The classical ketogenic diet is the established treatment of choice for:
pyruvate dehydrogenase deficiency, where the diet overcomes the deficiencies in a catalytic component of the mitochondrial enzyme pyruvate dehydrogenase complex by providing an alternative source of acetyl coenzyme A;
glucose transporter 1 deficiency syndrome, where the diet overcomes the impaired glucose transport across the blood–brain barrier by using ketone bodies as fuel (they do not rely upon the glucose transporter system to enter the brain). It is important to note that there are likely to be adults with glucose transporter 1 deficiency syndrome who are yet to be diagnosed and may be suitable for treatment with these diets.
In other epilepsies due to structural/metabolic, genetic or unknown causes, ketogenic dietary therapies have traditionally been reserved as a last resort, typically after the failure of ≥3 antiepileptic medications.31 Perhaps dietary therapy should be considered as an earlier treatment option in drug-resistant epilepsy.31 ,32 Introducing these diets in children who were treatment naïve or who had only tried one antiepileptic medication was as effective as in other patients with drug-resistant epilepsy, and with no adverse effects.33 There are certain syndromes/conditions where these diets appear particularly effective and so it may be appropriate to introduce them early:31 ,34 epilepsy with myoclonic atonic seizures, infantile spasms, mitochondrial respiratory chain defects, Lennox–Gastaut syndrome and Dravet's syndrome. There is also evidence that starting ketogenic dietary therapies help acutely in adults with status epilepticus who are not responding to antiepileptic medications;35–37 these reports include both enteral and parental feeding with ketogenic dietary therapies.
Starting the diet
Before starting a ketogenic dietary therapy, patients need screening (biochemical testing of blood and/or urine) for disorders of fatty acid metabolism and organic acidurias that may lead to deterioration when lipids are used as the primary energy source or when dietary intake of protein or certain amino acids is not restricted. Box 1 lists these disorders.
Disorders that contraindicate the use of ketogenic dietary therapy, taken from the International Ketogenic Diet Study Group consensus statement31
Disorders
Carnitine deficiency (primary)
Carnitine palmitoyltransferase I or II deficiency
Carnitine translocase deficiency
β-Oxidation defects
Medium-chain acyl dehydrogenase deficiency
Long-chain acyl dehydrogenase deficiency
Short-chain acyl dehydrogenase deficiency
Long-chain 3-hydroxyacyl-coenzyme A deficiency
Medium-chain 3-hydroxyacyl-coenzyme A deficiency.
Pyruvate carboxylase deficiency
Porphyria
Type 2 diabetes mellitus is not a contraindication to starting ketogenic dietary therapies. There are case reports of children with type 1 diabetes mellitus who have successfully followed these diets for their epilepsy,38 but no reports in adults.
There is a list of suggested biochemical analyses to be taken before starting ketogenic dietary therapies in children.31 For adults, we suggest (as a minimum) testing urine organic acids and serum lipids at baseline. Basic liver function and renal function tests may also be important in people taking antiepileptic medications related to the carbonic anhydrase inhibitor group. It is worth checking a full blood count and serum vitamin D concentrations to exclude deficiency. The extent of biochemical analyses depends on the patient's overall health.
In the preliminary consultation, it is important to assess other factors, such as possible patient or carer non-adherence, or patient's inability to maintain adequate nutrition. We do not know the effects of these diets during pregnancy, but extrapolating from animal studies39 we recommend avoiding pregnancy in adults starting a ketogenic dietary therapy. Many other factors should prompt caution before starting such diets, including a family history of hyperlipidaemia, nephrolithiasis or severe gastro-oesophageal reflux. These do not necessarily contraindicate the diet but the clinician should discuss the risks and potential benefits with the patient. Food allergies, intolerances and cultural/religious preferences should not prevent patients starting the diet.
The modified Atkins diet is probably the most suitable for adults, due to its use of household measures and unrestricted protein. However, in our experience, adults can follow either the classical ketogenic diet (in tube-fed and orally fed patients) or the modified Atkins diet. The suitability of a particular diet is highly patient specific. Often, there are blurred boundaries between the different types of diet; for example, patients may follow a modified Atkins-type diet but with additional medium-chain triglyceride oil, to achieve optimal efficacy and tolerability. Ketogenic dietary therapies are one of the few treatments that allow patients a feeling of empowerment over their condition; clinicians should work with patients to promote flexibility and independence, while still ensuring safety and adherence. Any adult ketogenic tube feed may be based on a classical ketogenic diet—working from a ketogenic ratio—or using a modification where the patient receives 20 g of carbohydrate, their protein requirements are met and the remaining calories are given as fat.
Before starting a ketogenic diet, the patient may complete a 3-day food diary to help the dietitian to calculate their daily energy requirement. A specialist dietitian and/or nurse might give a teaching session to adults and, if applicable, carers or family members. The advice given depends on the type of diet to be followed but normally covers the following: principles of ketogenic dietary therapies, home monitoring of ketones in blood or urine, frequency of future clinic appointments and required blood tests, the diet team's contact details, carbohydrate-free medications, ketogenic recipes, food exchange lists, free foods (if appropriate), potential adverse effects, what to do in case of illness and/or needing to be nil by mouth and emergency advice—for example, recognising and treating hyperketosis, metabolic acidosis or hypoglycaemia.
In the UK, adults are not usually admitted to start these diets and they use a non-fasting protocol. As soon as patients start the diet they should take a multivitamin, mineral and trace element supplement (this does not include treatment of deficiencies, eg, vitamin D or folate), plus 500–800 mg calcium, 200–300 mg magnesium and 10–20 μg vitamin D. Most adults have to buy their own supplements over the counter. All their medications should be carbohydrate-free.
Monitoring and maintenance
During the first few weeks on the diet, patients should liaise frequently with the dietitian to fine-tune the diet to achieve optimal ketosis, adherence and management of adverse effects. Depending on the centre, the patient may send in twice-daily blood or urinary ketone results, depending on the type of diet. The patient usually then has medical and dietetic reviews at 3 and 6 months, and 6-monthly thereafter. Patients should have repeat biochemical analyses at each follow-up appointment. The clinician should also review the patient's ketone results taken since the previous appointment; as the patient becomes more familiar with the diet and their symptoms, these may be tested less frequently. Patient and clinician should decide together how often to measure ketone levels at home, and for how long, depending on the patient's lifestyle and seizure control. Many paediatric centres recommend maintaining blood β-hydroxybutyrate concentrations at 4–6 mmol/L, but this is not based on clinical evidence; some people can achieve optimal seizure control with lower ketone levels. There are currently no recommendations for optimal ketone levels in adults.
It is customary to follow the diet for 3 months before considering stopping it, unless seizures worsen.31 Preferably, patients should not change other antiepileptic medications during this time to make it easier to assess the diet's effect. At each consultation, the patient and the multidisciplinary team should discuss whether the diet is meeting previously agreed expectations (eg, a certain level of seizure reduction, or reduced need for emergency seizure medications).
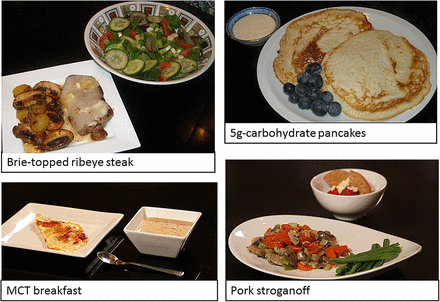
In time, patients may wish to be more adventurous and branch out from the prescribed ketogenic meals and snacks: figure 2 gives examples of these. Charities dedicated to supporting patients and their families have several sources of recipes and information on these diets, and there are other publications. Box 2 lists some resources. People may also wish to create their own recipes using specialised software, such as EKM (Electronic Ketogenic Manager) or myKetoPlan (http://ketoplanner.edm2000.com), with close medical and dietetic supervision.
Ketogenic dietary therapy resources41–44
Matthew's Friends charity: http://www.matthewsfriends.org
The Charlie Foundation: http://www.charliefoundation.org
The Daisy Garland charity: http://www.thedaisygarland.org.uk/the-ketogenic-diet-2
Epilepsy Society: http://www.epilepsysociety.org.uk/ketogenic-diet
Examples of ketogenic meals and snacks. Pictures provided by Emma Williams, Matthew's Friends charity. MCT, medium-chain triglyceride.
Adults with hyperlipidaemia who are responding well to ketogenic dietary therapies may consider increasing fat sources of omega 3, such as fish oil supplements, and adding flax or walnut oil. There are published ‘Heart Healthy’ recommendations for the modified Atkins diet.22 Patients might also substitute medium-chain triglyceride fats for long-chain triglyceride fats, or take L-carnitine supplements.
Stopping dietary treatment
Patients should usually follow a ketogenic dietary therapy for 2 years, if effective and tolerated, before considering stopping it. This is based on the common practice of stopping antiepileptic medication at this point in children who become seizure-free. However, the diets may be continued for much longer than 2 years, for example, in people with glucose transporter-1 deficiency syndrome.
Eighty per cent of children who become seizure-free on ketogenic dietary therapies remain so after stopping the diet.40 Only one study has evaluated the response in adults to stopping the diet: seizure improvement did not outlast treatment.21 We need further studies to determine the optimal duration of dietary treatment and whether its antiepileptic effects continue after it stops.
There is no consensus on the best way to stop these diets; it is usually done over several weeks or months on an individual basis, depending on their response; patients should monitor ketones until returning to a normal diet. It is best to withdraw dietary treatment particularly slowly in those who have followed the diet for a long time or whose seizure frequency dramatically reduced when taking it. For example, patients can reduce the ketogenic ratio of the classical ketogenic diet by 0.25, 0.5 or 1.0 at a time, can change a ketogenic meal/snack to a regular snack one at a time or may reduce the number of fat choices by 1–2 per day or every couple of days. Patients should only reintroduce foods rich in refined carbohydrates after returning to a normal diet.
Adults may be apprehensive about stopping treatment, particularly if they have had a favourable response, and so often need ongoing support during this period. If seizure frequency increases during weaning, the patient should discuss with the ketogenic diet team whether to take the withdrawal more slowly or whether to remain on the diet. When modifying or discontinuing ketogenic dietary therapies, the same Driver and Vehicle Licensing Agency guidance applies as when changing antiepileptic medication.
Key points
Ketogenic dietary therapies can be an effective treatment option for adults with drug-resistant epilepsy, although controlled studies are needed.
Adverse effects appear similar to those reported in children, and are usually non-critical and/or transient; short-term use of these diets does not detrimentally affect lipid profiles.
Patients need a variety of biochemical tests before starting these diets; clinicians should discuss the risks and benefits of the diet and identify potential barriers to adherence.
Regular medical and dietetic monitoring is essential throughout dietary treatment, although there are many available resources to help patients become more independent and adventurous with their diet as their confidence increases.
Acknowledgments
We thank Susan Wood, Adult Ketogenic Dietitian from Matthew's Friends charity, for information provided regarding adult ketogenic dietary therapy services in the UK and valuable practical advice on diet administration in adults; also to Emma Williams from Matthew's Friends charity for kindly providing pictures for use in this manuscript.
References
Footnotes
Contributors NES and JHC both drafted and revised the paper.
Funding Vitaflo; Nutricia.
Competing interests JHC has received funds to the department for research into the ketogenic diet from Vitaflo. Honoraria for speaking have also been made to the department on her behalf from Nutricia. JHC has co-written a cookery book, ‘Ketocooking’, funds from the sale of which will be donated to the department. JHC currently receives funds for ketogenic diet trials from the European Union (FP7) and NIHR.
Provenance and peer review Commissioned; externally peer reviewed. This paper was reviewed by Rhys Thomas, Cardiff and Manny Bagary, Birmingham, UK.
Linked Articles
- Editors' commentary
Other content recommended for you
- Ketogenic diets in the treatment of epilepsy
- Efficacy of medium chain triglyceride oil dietary supplementation in reducing seizure frequency in dogs with idiopathic epilepsy without cluster seizures: a non-blinded, prospective clinical trial
- What is a ketogenic diet and how does it affect the use of medicines?
- Genetic variants for personalised management of very low carbohydrate ketogenic diets
- Treatment of difficult epilepsy
- Alternative approaches to conventional antiepileptic drugs in the management of paediatric epilepsy
- The ketogenic diet in childhood epilepsy: where are we now?
- Fifteen-minute consultation: When medicines don’t work—the child with poorly controlled seizures
- Question 1 Efficacy of the ketogenic diet in difficult childhood epilepsies
- Glut1 deficiency syndrome: Absence epilepsy and La Soupe du Jour