Article Text
Abstract
Autonomic dysreflexia is a relatively common condition in people who have a spinal cord injury above the level of T6. It is a potentially life-threatening; without timely and effective treatment, it can have deleterious cardiophysiological and systemic consequences. It is therefore imperative for medical professionals to have a clear understanding of its acute management, and be prepared to provide support and education to those caring for at-risk patients. In this paper we provide practical guidance and supporting evidence regarding the management of autonomic dysreflexia in adults with spinal cord injury.
- rehabilitation
- clinical neurology
- autonomic
Statistics from Altmetric.com
Introduction
Autonomic dysreflexia is, by definition, an acute increase in systolic blood pressure (BP), usually by at least 20 mm Hg, associated with baroreflex-mediated bradycardia. It results from unmodulated sympathetic reflex activity triggered by common and usually reversible factors, most commonly bladder or bowel distension. People with spinal cord injury at or above the level of T6 are most at risk. It is a potentially life-threatening complication of spinal cord injury1; if not treated properly and promptly, it can have deleterious cardiophysiological and systemic consequences,2 including seizures, stroke and intracerebral haemorrhage.3–6
Most episodes of autonomic dysreflexia are managed at home or in the community by the patient and/or their trained carer and can be resolved without acute medical support. Only rarely is urgent escalation required to treat severe, high risk or treatment-resistant episodes. Consequently, those who do not work in specialist centres may rarely see the condition and may not be trained in its acute management.
When someone with chronic spinal cord injury is admitted to hospital, or more rarely in newly diagnosed cases, there is a risk that autonomic dysreflexia will occur without there being immediately available specialist support. This may delay both diagnosis and appropriate management.7 It is therefore imperative for medical professionals to have a clear understanding of the acute management of autonomic dysreflexia and to able to provide support and education to others caring for at-risk patients.
Background
Autonomic dysreflexia is relatively common in people with spinal cord injuries above the level of T6. It is most often managed outside of the hospital environment, so published figures of its prevalence vary widely, between 48% and 70%.8 It is three times more common in those with complete spinal cord injury (91%) than in those with incomplete injury (27%).9
Ninety-two per cent of people who develop autonomic dysreflexia do so in their first year after their injury.10 It generally occurs only after the spinal shock phase of cord injury, when the reflexes have returned.11 At this stage, the recovery of neuronal excitability within the spinal cord is unopposed by descending (supraspinal) modulation, giving rise to sympathetic hyperreflexia below the lesion. This is analogous to spasticity in the motor system.
Causes
Autonomic dysreflexia occurs as a response to a (usually noxious) stimulus below the level of the spinal cord injury.3 Recurrent episodes of autonomic dysreflexia in the absence of obvious trigger factors may in itself serve as an important indicator of the presence of undetected underlying pathology.12
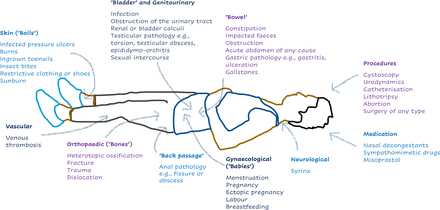
The most common cause is bladder over-distension, accounting for 75%–85% of episodes.13 The second most common cause is over-distension of the bowel: in a study investigating the influence of autonomic dysreflexia on bowel management in the community after spinal cord injury, 74% of 287 respondents experienced autonomic dysreflexia symptoms during bowel care.14 The common causes can be remembered as the ‘six Bs’: bladder (urinary tract infection, retention, stones, blocked catheters), bowel (constipation, impaction), back passage (haemorrhoid or fissure), boils (skin damage), bones (fractures) and babies (pregnancy, sexual intercourse, breast feeding).1 Figure 1 lists other causes to consider.
The causes of autonomic dysreflexia by system.
The pathophysiology
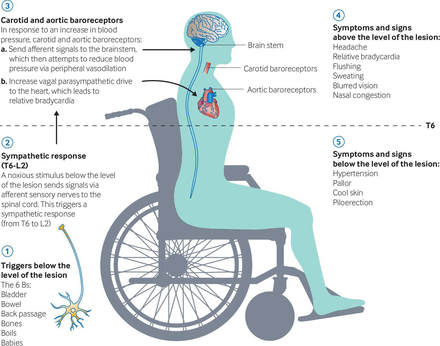
An appreciation of the pathophysiology underlying autonomic dysreflexia is essential for understanding of the clinical features, trigger factors and potential sequalae of this condition.8 A noxious stimulus sends signals via afferent sensory nerves to the spinal cord. This triggers a sympathetic response (from T6 to L2) that is pathologically exaggerated, due to intrinsic cord hyperexcitability below the lesion and a lack of descending modulation. This causes massive vasoconstriction of the muscular, splanchnic and cutaneous vascular beds.8
The increase in BP stimulates the carotid and aortic baroreceptors, significantly increasing the brainstem’s parasympathetic outflow. The resulting descending vagal drive causes relative bradycardia and peripheral vasodilatation. However, this vagal drive cannot pass below the level of the lesion; the unopposed sympathetic activity below that level can lead to a life-threatening rise in BP and its sequelae6 if the stimulus is not promptly recognised and withdrawn. Patients with spinal injuries below T10 rarely experience autonomic dysreflexia, as the splanchnic innervation remains intact and allows for compensatory parasympathetic dilatation of the splanchnic vascular bed.3
Symptoms and signs
Patients frequently report a sudden onset and severe, throbbing headache. The headache is classically bilateral and located in the frontal, temporal or occipital regions.12 There may be flushing and sweating of the skin above the level of their lesion, alongside pupillary constriction, blurred vision and nasal congestion. Sympathetic overdrive causes pallor, cool skin and piloerection below the level of the lesion.15 Other common features include cutis anserina (goose bumps), spasticity, general malaise and dizziness.14
The symptoms do not necessarily correlate with the BP. Patients with spinal cord injury above T6 tend to have a systolic BP between 90 and 110 mm Hg.11 Moreover, patients with cervical spinal cord injury commonly already have orthostatic hypotension. Patients with symptoms of autonomic dysreflexia may have a normal (or only modestly raised) BP: it is therefore important where possible to compare their BP to baseline.
Figure 2 outlines the pathophysiology and clinical presentation of autonomic dysreflexia.
The pathophysiology and clinical presentation of autonomic dysreflexia. Reproduced with permission from Cowan et al. 2
How to treat it
Acute management
It is important as soon as possible to ask the patient and their carer(s) about their common precipitating factors and usual management. Patients at risk of autonomic dysreflexia are usually well-educated in this condition and have a self-management plan, which includes at all times carrying a rescue pack containing the appropriate medications1 (organised by their specialist team) (figure 3).
The acute management of autonomic dysreflexia. BP, blood pressure.
As soon as practically possible, the carer or clinician should reposition the patient into an upright position as this promotes an orthostatic drop in BP, and also loosen all tight clothing, dressings, catheter leg bags and plaster casts.12 It is then important to rule out systematically and to manage any potential triggers. In most cases, a thorough patient review negates the need for escalation to pharmacological management.
Distension of the bladder should be suspected as the most likely cause, and the patient examined for a palpable bladder. If they have a catheter, it should be repositioned, unkinked and flushed. The flushing should be done with 0.9% saline, ideally warmed to body temperature, as cold irrigation could act as a further trigger for autonomic dysreflexia.12 If there is a suspected ongoing blockage, the catheter should be changed immediately. If the patient does not have a catheter, then insert one with lignocaine gel (which is thought to prevent exacerbation of autonomic dysreflexia). The rapid emptying of the bladder can precipitate sudden hypotension, particularly if the patient has already received medication. The BP should therefore be monitored during bladder emptying. If the bladder is over-distended, drain 500 mL initially, then 250 mL every 10–15 min to avoid reactive hypotension.
The BP should be monitored every 2–5 min.11 If the systolic BP is elevated to 40 mm Hg above patient’s baseline level, or the systolic BP is ≥150 mm Hg, start pharmacological management before assessing for other potential triggers.
Use an antihypertensive agent with rapid onset and short duration to manage acute episodes of autonomic dysreflexia.
Nifedipine 10 mg in the immediate release capsule form is usual first-line treatment, delivered using the ‘bite and swallow’ method. Prescribers may wish to consider reducing the dose to 5 mg in the elderly or those with concomitant prescriptions of other antihypertensive agents, particularly beta-blockers. The sublingual form is not recommended as it has an unknown rate of absorption. Repeat the treatment every 20–30 min if required to a maximum of 40 mg in 24 hours.
Nitrates are the second most commonly used agents, but there is little published evidence to show their efficacy or safety in the spinal cord injury population. If the patient has taken sildenafil or other cGMP-specific phosphodiesterase type 5 inhibitors within 24 hours of the episode occurring, then nitrates are contra-indicated, as they may enhance hypotension. The options for nitrate treatment:
Glyceryl trinitrate (GTN) 400 mg spray, given as one spray sublingually, repeated after 5–10 min (maximum of three sprays in 15 min).
GTN 500 mg sublingual tablet. Held under the tongue (mouth mucosa must be moist) repeated after 5–10 min (maximum three in 15 min).
GTN 5 mg patch, which can be removed once the BP has returned to baseline.
These medications carry a risk of rebound hypotension, which can continue for up to 5 hours following administration.16 All drugs should therefore be used with caution and in conjunction with the non-pharmacological methods described above.
Patients with spinal cord injury often already have orthostatic hypotension, making it important to titrate medication carefully in the acute setting and to monitor BP closely. Moreover, the BP may fluctuate rapidly during an acute episode owing to the impaired autonomic regulation associated with chronic spinal cord injury.12 In all cases, continue to monitor the patient’s BP for at least 2 hours after an episode to ensure there is no rebound hypotension,11 or longer in severe cases. This should not detract from using these medications in this potentially life-threatening condition but reinforces the need to monitor BP carefully after giving them. If the BP remains elevated despite review and management of the bladder, but is less than 150 mm Hg systolic, then next consider possible bowel-related causes. Examine the patient’s abdomen and perform a digital rectal examination using lignocaine gel. Manually evacuate palpable stool using lignocaine gel and gentle digital stimulation.11 A suppository can be used if required. Generally, avoid large volume enemas or excessively vigorous digital stimulation, as these may exacerbate the condition.17 If the BP increases during these manoeuvres, stop to insert more topical lidocaine/chlorhexidine 2%/0.25% gel; recheck the BP and consider treating with an antihypertensive agent. Re-examine the rectum again for the presence of stool after 20 min and remove this gently if found.
Patients should be warned of the potential side effects of treatment. With both classes of drug, common adverse effects are: arrhythmias, tachycardia, dizziness, drowsiness, flushing, headache, nausea or vomiting. Note, however, that the patient’s clinical presentation may mask some of these. If the cause of the episode has not yet been found then next make a detailed and systematic examination, while considering the other potential triggers. The other relatively common culprits include infected or ingrown toenails, infected pressure sores, previously unidentified dislocation of the hip, fracture, heterotopic ossification or deep venous thrombosis. Figure 1 can be used as a guide to work through: consider these using a systems-based approach.
Admit the patient to hospital if they respond poorly to the above treatment, or have no identified cause, or have a suspected obstetric complication.11
Evaluate the effectiveness of the interventions, keeping in mind the points below:
The cause for the episode should ideally have been identified. Autonomic dysreflexia may resolve because of medication, but not because the underlying cause has resolved. Unless the cause has been identified and addressed, recurrence should be expected.
The patient has been assessed for the sequalae of autonomic dysreflexia, including raised intracranial pressure or heart failure.
The BP should return to the patient’s normal level and remain stable.
If the episode does not settle with the above management, or has not resolved after 30 min, consider transfer and escalation to intensive care for further management. This should also be considered in cases with suspected or existing end-organ damage secondary to hypertension. The patient may require intravenous treatment and careful down-titration of BP in a monitored setting (see box 1 for options, although the choice will vary depending on local availability). Inform the specialist spinal rehabilitation team at the earliest opportunity who will provide ongoing advice and support.
Intravenous options for refractory hypertension in autonomic dysreflexia
Phentolamine (5 mg intravenously prn): a non-selective α1 adrenergic antagonist.
Clonidine: an α2 agonist acts at centrally on α2 receptors in the brainstem, reduces peripheral vascular resistance and lowers BP. Clonidine is also an agonist at imidazoline-1 (I1) receptors and acts to decrease sympathetic signalling.
Hydralazine (10–20 mg intravenously slowly prn): the precise mechanism of action is not fully understood, but the major effects are on the cardiovascular system. Hydralazine appears to lower BP by causing peripheral vasodilation in resistance arterioles through a mechanism involving inhibition of inositol triphosphate-induced calcium release from the sarcoplasmic reticulum in arterial smooth muscle. This results in direct relaxation of arteriole smooth muscle and a decrease in peripheral resistance, blood pressure and afterload.
Sodium nitroprusside (0.5–3 mcg/kg/min prn): this is broken down in the circulation to nitric oxide, resulting in potent vasodilating effects of arterioles more than venules. Nitric oxide activates guanylate cyclase in vascular smooth muscle and increases intracellular production of cGMP activating protein kinase G and phosphatases, which inactivate myosin light chains involved in muscle contraction. The end result is vascular smooth muscle relaxation, allowing vessels to dilate.
Diazoxide (20 mg intravenous bolus) is a potassium channel activator and enhances cell membrane permeability to potassium ions. This action elicits the relaxation of local smooth muscles.
Inpatients should have their BP monitored for 2 hours following an episode of autonomic dysreflexia.11 If the BP is well-controlled and serious acute causes have been ruled out, other investigations can be done on an outpatient basis. Follow-up should continue until a precipitating cause is found or the episodes cease.18 The patient should be informed that for a few days following an episode, they will have a lower threshold for precipitation of further episodes.16
Patients with frequent severe occurrences of autonomic dysreflexia during the rehabilitation phase should only leave the unit with appropriately trained staff in attendance. Decisions to allow patients to leave a unit should be made in collaboration with medical staff. The patient, carers and staff should take the supplies necessary for treating autonomic dysreflexia when on leave from the unit and on discharge home.
Long-term management
The long-term management of patients with spinal cord injuries is overseen by a spinal rehabilitation consultant and multidisciplinary team. Once the patient is ready for discharge from acute hospital care following spinal injury, it is appropriate to refer them to this team.
For someone at risk of autonomic dysreflexia, part of the management is its prevention. This includes the provision of a bladder and bowel management programme and a skin integrity programme.
The team should investigate and manage urinary tract related complications and treat coexisting infection. The method and type of management should be tailored to the individual and may be overseen by a neuro-urologist. If a patient conducts intermittent self-catheterisation long term, we recommend that the bladder is emptied at least 5–6 times every 24 hours to prevent bladder overdistension. Indwelling catheters should be changed regularly in line with spinal injury team guidance.
A bowel management regimen should be put in place, again with specialist spinal team guidance. Management involves non-pharmacological methods (abdominal massage and digital rectal stimulation) and medications (daily suppositories and laxatives).17 The removal of faeces may itself potentially trigger autonomic dysreflexia and therefore clinicians should use topical lignocaine jelly for digital rectal stimulation, examination or evacuation. The above measures, when followed as a part of a normal daily routine, should prevent episodes of faecal impaction and severe constipation occurring.
Skin integrity is promoted with positioning and seating guidelines and the use of pressure-relieving mattresses and cushions. The patient’s nutritional status should also be optimised. It is important to detect and manage pain, and to remove its possible sources, as in some cases a patient’s only sign of pain may be the experience of autonomic dysreflexia episodes.
If a patient in the community with spinal cord injury experiences recurrent episodes despite the above management, a specialist spinal rehabilitation consultant’s opinion should be sought to investigate the less common triggers of autonomic dysreflexia, for example, syrinx, gallstones or bladder or renal calculi. They will also be able to provide advice on how to prevent and manage autonomic dysreflexia episodes before elective procedures, and during pregnancy and labour.
In terms of pharmacological management, prescribing a daily anti-hypertensive medication is not recommended for treating autonomic dysreflexia long term as patients often have co-existing orthostatic hypotension. Other risk factors for cardiovascular disease should be investigated and treated as required.
Patient and carer education are at the heart of all management, as it is they who best understand their condition and will be managing most autonomic dysreflexia episodes.
Illustrative case study
Case 1
A 63-year old woman with rheumatoid arthritis and taking long-term corticoosteroids, developed C7–T1 osteoporotic fractures, which resulted in cord compression. She underwent posterior decompression and C3–T4 cervico-thoracic fixation but postoperatively she was diagnosed with a C6 incomplete spinal cord injury. During postoperative rehabilitation, she complained of excessive sweating and light-headedness associated with recurrent episodes of autonomic dysreflexia.
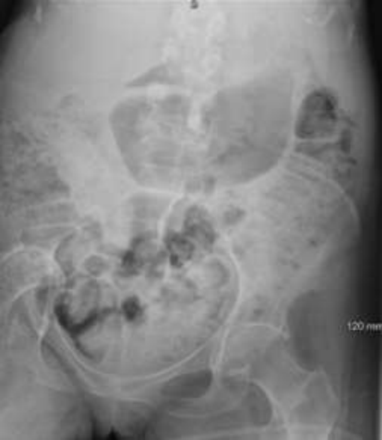
She was systematically examined and investigated for the cause. At that time, she had a long-term indwelling catheter that was draining well, and urinary tract infection had been ruled out. Her skin was examined for pressure areas. An abdominal X-ray identified impacted bowels with associated chronic distension of the transverse colon, and there was an incidental finding of right hip avascular necrosis protrusio acetabuli (figures 4 and 5).
X-ray of abdomen showing extensive faecal impaction throughout the colon alongside right hip avascular necrosis and protrusio acetabuli.
X-ray of abdomen showing extensive faecal impaction throughout the colon alongside right hip avascular necrosis and protrusio acetabuli.
The episodes of autonomic dysreflexia resolved with laxatives, enemas and a daily bowel regimen. Although initially a consideration, the hip pathology was considered to be longstanding and not contributing to her episodes. Given her overall presentation and recovery trajectory, the hip was managed conservatively.
Key points
Autonomic dysreflexia is an acute increase in systolic blood pressure (BP) (above 20 mm Hg from baseline) with headache, and occurs in people with spinal cord injury above the level T6.
Its common causes are ‘6 Bs’: bladder (urinary tract infection, retention, stones, blocked catheters), bowel (constipation, impaction), back passage (haemorrhoid or fissure), boils (skin damage), bones (fractures) and babies (pregnancy, sexual intercourse, breast feeding).
The mainstay of its acute management involves systematically identifying and treating these triggers, followed by BP-lowering medications if the systolic is ≥150 mm Hg, or is ≥40 mm Hg above baseline.
Consider transfer and escalation to intensive care if the episode persists beyond 30 min, or where there is existing or suspected end-organ damage secondary to hypertension.
Patients at risk of autonomic dysreflexia require long-term management including bladder, bowel and skin routines, overseen by the patient’s spinal rehabilitation team.
Further reading
Consortium for Spinal Cord Medicine. Acute management of autonomic dysreflexia: individuals with spinal cord injury presenting to healthcare facilities. J Spinal Cord Med. 2002;25(Suppl 1):S67-88. This document provides a step-by-step guide for the acute management of AD. It also lists the rarer triggers of AD episodes, which should be considered if the most common causes are ruled out. Lastly, it provides guidance on the management for pregnant women, children and adolescents, which fell beyond the scope of this article.
Eldahan KC, Rabchevsky AG. Autonomic dysreflexia after spinal cord injury: Systemic pathophysiology and methods of management. Auton Neurosci. 2018;209:59–70. This paper provides an evidence-based explanation of the theory of the mechanisms contributing to AD, and its treatments. The authors describe the effects of recurrent episodes on cardiovascular and immune functions and suggest further avenues for research into the area.
http://www.elearnsci.org/. This website provides evidence-based information for both patients and medical professionals on the subject of spinal cord injury. In particular, the modules provide detailed and accessible information on the management of all problems related to spinal injury, including many of the topics mentioned in this paper.
Ethics statements
Patient consent for publication
References
Footnotes
Contributors The authors of this paper have all contributed significantly and fairly to the writing and/or editing. There have been no additional contributors to the paper.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Commissioned. Externally peer reviewed by Sree Kolli, Cardiff, UK.
Linked Articles
- Editors’ commentary
Other content recommended for you
- Medical emergency: rash, headache and spinal cord injury
- Protocol for a phase II, open-label exploratory study investigating the efficacy of fesoterodine for treatment of adult patients with spinal cord injury suffering from neurogenic detrusor overactivity for amelioration of autonomic dysreflexia
- Autonomic dysreflexia: a medical emergency
- Organisation of the sympathetic skin response in spinal cord injury
- Effects of exercise interventions on cardiovascular health in individuals with chronic, motor complete spinal cord injury: protocol for a randomised controlled trial [Cardiovascular Health/Outcomes: Improvements Created by Exercise and education in SCI (CHOICES) Study]
- Health promotion and cardiovascular risk reduction in people with spinal cord injury: physical activity, healthy diet and maintenance after discharge— protocol for a prospective national cohort study and a preintervention- postintervention study
- Effect of wheelchair-modified rowing exercise on cardiometabolic risk factors in spinal cord injured wheelchair users: protocol for a randomised controlled trial
- Knowledge of autonomic dysreflexia in the emergency department
- Testing for boosting at the Paralympic games: policies, results and future directions
- Effect of epidural spinal cord stimulation after chronic spinal cord injury on volitional movement and cardiovascular function: study protocol for the phase II open label controlled E-STAND trial