Abstract
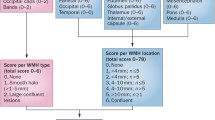
White matter changes occur endemically in routine magnetic resonance imaging (MRI) scans of elderly persons. MRI appearance and histopathological correlates of white matter changes are heterogeneous. Smooth periventricular hyperintensities, including caps around the ventricular horns, periventricular lining and halos are likely to be of non-vascular origin. They relate to a disruption of the ependymal lining with subependymal widening of the extracellular space and have to be differentiated from subcortical and deep white matter abnormalities. For the latter a distinction needs to be made between punctate, early confluent and confluent types. Although punctate white matter lesions often represent widened perivascular spaces without substantial ischemic tissue damage, early confluent and confluent lesions correspond to incomplete ischemic destruction. Punctate abnormalities on MRI show a low tendency for progression, while early confluent and confluent changes progress rapidly. The causative and modifying pathways involved in the occurrence of sporadic age-related white matter changes are still incompletely understood, but recent microarray and genome-wide association approaches increased the notion of pathways that might be considered as targets for therapeutic intervention. The majority of differentially regulated transcripts in white matter lesions encode genes associated with immune function, cell cycle, proteolysis, and ion transport. Genome-wide association studies identified six SNPs mapping to a locus on chromosome 17q25 to be related to white matter lesion load in the general population. We also report first and preliminary data that demonstrate apolipoprotein E (ApoE) immunoreactivity in white matter lesions and support epidemiological findings indicating that ApoE is another factor possibly related to white matter lesion occurrence. Further insights come from modern MRI techniques, such as diffusion tensor and magnetization transfer imaging, as they provide tools for the characterization of normal-appearing brain tissue beyond what can be expected from standard MRI scans. There is a need for additional pre- and postmortem studies in humans, including these new imaging techniques.




Similar content being viewed by others
References
Atwood LD, Wolf PA, Heard-Costa NL et al (2004) Genetic variation in white matter hyperintensity volume in the Framingham Study. Stroke 35:1609–1613
Auriel E, Bornstein NM, Berenyi E et al (2011) Clinical, radiological and pathological correlates of leukoaraiosis. Acta Neurol Scand 123:41–47
Awad I, Johnson P, Spetzler R, Hodak J (1986) Incidental subcortical lesions identified on magnetic resonance imaging in the elderly. II. Postmortem pathological correlations. Stroke 17:1090–1097
Baezner H, Blahak C, Poggesi et al (2008) Association of gait and balance disorders with age-related white matter changes: the LADIS study. Neurology 70:935–942
Barber R, Scheltens P, Gholkar A et al (1999) White matter lesions on magnetic resonance imaging in dementia with Lewy bodies, Alzheimer’s disease, vascular dementia, and normal aging. J Neurol Neurosurg Psychiatry 67:66–72
Basile AM, Pantoni L, Pracucci G et al (2006) Age, hypertension, and lacunar stroke are the major determinants of the severity of age-related white matter changes. The LADIS (Leukoaraiosis and Disability in the Elderly) Study. Cerebrovasc Dis 21:315–322
Bastin ME, Clayden JD, Pattie A, Gerrish IF, Wardlaw JM, Deary IJ (2009) Diffusion tensor and magnetization transfer MRI measurements of periventricular white matter hyperintensities in old age. Neurobiol Aging 30:125–136
Benedetti B, Charil A, Rovaris M et al (2006) Influence of aging on brain gray and white matter changes assessed by conventional, MT, and DT MRI. Neurology 66:535–539
Bhadelia RA, Price LL, Tedesco KL et al (2009) Diffusion tensor imaging, white matter lesions, the corpus callosum, and gait in the elderly. Stroke 40:3816–3820
Braffman B, Zimmerman R, Trojanowski J, Gonatas N, Hickey W, Schlaepfer W (1988) Brain MR: pathologic correlation with gross and histopathology. 1. Lacunar infarction and Virchow–Robin spaces. AJNR Am J Neuroradiol 151:551–558
Bronge L, Bogdanovic N, Wahlund LO (2002) Postmortem MRI and histopathology of white matter changes in Alzheimer brains. A quantitative, comparative study. Dement Geriatr Cogn Disord 13:205–212
Carmelli D, DeCarli C, Swan GE et al (1998) Evidence for genetic variance in white matter hyperintensity volume in normal elderly male twins. Stroke 29:1177–1181
Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG (2009) CADASIL. Lancet Neurol 8:643–653
Chabriat H, Pappata S, Poupon C et al (1999) Clinical severity in CADASIL related to ultrastructural damage in white matter: in vivo study with diffusion tensor MRI. Stroke 30:2637–2643
Chimowitz MI, Estes ML, Furlan AJ, Awad IA (1992) Further observations on the pathology of subcortical lesions identified on magnetic resonance imaging. Arch Neurol 49:747–752
Desmond DW (2002) Cognition and white matter lesions. Cerebrovasc Dis 13(Suppl 2):53–57
Erkinjuntti T, Benavente O, Eliasziw M et al (1996) Diffuse vacuolization (spongiosis) and arteriolosclerosis in the frontal white matter occurs in vascular dementia. Arch Neurol 53:325–332
Fazekas F, Chawluk J, Alavi H, Hurtig H, Zimmerman R (1987) MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJNR Am J Neuroradiol 8:421–426
Fazekas F, Kapeller P, Schmidt R, Offenbacher H, Payer F, Fazekas G (1996) The relation of cerebral magnetic resonance signal hyperintensities to Alzheimer’s disease. J Neurol Sci 142:121–125
Fazekas F, Kleinert R, Offenbacher H et al (1991) The morphologic correlate of incidental punctate MR white matter hyperintensities. AJNR Am J Neuroradiol 12:915–921
Fazekas F, Kleinert R, Offenbacher H et al (1993) Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology 43:1683–1689
Fazekas F, Ropele S, Enzinger C (2005) MTI of white matter hyperintensities. Brain 128:2926–2932
Fernando MS, O’Brien JT, Perry RH et al (2004) Comparison of the pathology of cerebral white matter with post-mortem magnetic resonance imaging (MRI) in the elderly brain. Neuropathol Appl Neurobiol 30:385–395
Fernando MS, Simpson JE, Matthews F et al (2006) White matter lesions in an unselected cohort of the elderly: molecular pathology suggests origin from chronic hypoperfusion injury. Stroke 37:1391–1398
Fornage M, Debette S, Bis JC et al (2011) Genome-wide association studies of cerebral white matter lesion burden: the CHARGE consortium. Ann Neurol 69:928–939
Gebril OH, Kirby J, Savva G, Brayne C, Ince PG et al (2011) HFE H63D, C282Y and AGTR1 A1166C polymorphisms and brain white matter lesions in the aging brain. J Neurogenet. doi:10.3109/01677063.2011.556206
Godin O, Tzourio C, Maillard P, Alperovitch A, Mazoyer B, Dufouil C (2009) Apolipoprotein E genotype is related to progression of white matter lesion load. Stroke 40:3186–3190
Gouw AA, Seewann A, van der Flier WM et al (2011) Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry 82:126–135
Gouw AA, Seewann A, Vrenken H et al (2008) Heterogeneity of white matter hyperintensities in Alzheimer’s disease: post-mortem quantitative MRI and neuropathology. Brain 131:3286–3298
Grafton ST, Sumi SM, Stimac GK, Alvord EC Jr, Shaw CM, Nochlin D (1991) Comparison of postmortem magnetic resonance imaging and neuropathologic findings in the cerebral white matter. Arch Neurol 48:293–298
Graham SJ, Henkelman RM (1997) Understanding pulsed magnetization transfer. J Magn Reson Imaging 7:903–912
Gunning-Dixon FM, Brickman AM, Cheng JC, Alexopoulos GS (2009) Aging of cerebral white matter: a review of MRI findings. Int J Geriatr Psychiatry 24:109–117
Hachinski VC, Potter P, Merskey H (1987) Leuko-araiosis. Arch Neurol 44:21–23
Henkelman RM, Stanisz GJ, Graham SJ (2001) Magnetization transfer in MRI: a review. NMR Biomed 14:57–64
Hennerici M, Baezner H, Daffertshofer M (2000) White matter changes: symptoms and signs. In: Pantoni L, Inzitari D, Wallin A (eds) The matter of white matter. Academic Pharmaceutical Productions, Utrecht, pp 55–80
Holland PR, Bastin ME, Jansen MA et al. (2010) MRI is a sensitive marker of subtle white matter pathology in hypoperfused mice. Neurobiol Aging doi:10.1016/j.neurobiolaging.2010.11.009
Inzitari D, Pracucci G, Poggesi A et al (2009) Changes in white matter as determinant of global functional decline in older independent outpatients: three year follow-up of LADIS (leukoaraiosis and disability) study cohort. BMJ 339:b2477
Jokinen H, Kaska H, Mäntylä R et al (2005) White matter hyperintensities as a predictor of neuropsychological deficits post-stroke. J Neurol Neurosurg Psychiatry 76:1229–1233
Jokinen H, Kalska H, Mäntylä R et al (2006) Cognitive profile of subcortical vascular disease. J Neurol Neurosurg Psychiatry 77:28–33
Jokinen H, Kalska H, Ylikoski R et al (2009) MRI-defined subcortical ischemic vascular disease: baseline clinical and neuropsychological findings. The LADIS study. Cerebrovasc Dis 27:336–344
Jokinen H, Kalska H, Ylikoski R et al (2009) Longitudinal cognitive decline in subcortical ischemic vascular disease. The LADIS Study. Cerebrovasc Dis 27:384–391
Jouvent E, Poupon C, Gray F (2011) Intracortical infarcts in small vessel disease: a combined 7-T postmortem MRI and neuropathological case study in cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Stroke 42:27–30
Lee DY, Fletcher E, Martinez O et al (2009) Regional pattern of white matter microstructural changes in normal aging, MCI, and AD. Neurology 73:1722–1728
Leifer D, Buonanno FS, Richardson EP (1990) Clinicopathologic correlations of cranial magnetic resonance imaging of periventricular white matter. Neurology 40:911–918
Mantyla R, Erkinjuntti T, Salonen O et al (1997) Variable agreement between visual rating scales for white matter hyperintensities on MRI. Comparison of 13 rating scales in a poststroke cohort. Stroke 28:1614–1623
Marshall V, Bradley WJ, Marshall C, Bhoopat T, Rhodes R (1988) Deep white matter infarction correlation of MR imaging and histopathologic findings. Radiology 167:517–522
Mori S, Zhang J (2006) Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 51:527–539
Mukherjee P, Berman JI, Chung SW, Hess CP, Henry RG (2008) Diffusion tensor MR imaging and fiber tractography: theoretic underpinnings. AJNR Am J Neuroradiol 29:632–641
Mukherjee P, Berman JI, Chung SW, Hess CP, Henry RG (2008) Diffusion tensor MR imaging and fiber tractography: technical considerations AJNR. Am J Neuroradiol 29:843–852
Munoz DG, Hastak SM, Harper B, Lee D, Hachinski VC (1993) Pathologic correlates of increased signals of the centrum ovale on magnetic resonance imaging. Arch Neurol 50:492–497
Nitkunan A, Charlton RA, McIntyre DJ, Barrick TR, Howe FA, Markus HS (2008) Diffusion tensor imaging and MR spectroscopy in hypertension and presumed cerebral small vessel disease. Magn Reson Med 59:528–534
O’Sullivan M, Morris RG, Huckstep B, Jones DK, Williams SC, Markus HS (2004) Diffusion tensor MRI correlates with executive dysfunction in patients with ischaemic leukoaraiosis. J Neurol Neurosurg Psychiatry 75:441–447
Pantoni L, Basile AM, Pracucci G et al (2005) Impact of age-related cerebral white matter changes on the transition to disability—the LADIS study: rationale, design and methodology. Neuroepidemiology 24:51–62
Pantoni L, Garcia JH (1995) The significance of cerebral white matter abnormalities 100 years after Binswanger’s report: a review. Stroke 26:1293–1301
Pfefferbaum A, Sullivan EV, Adalsteinsson E, Garrick T, Harper C (2004) Postmortem MR imaging of formalin-fixed human brain. Neuroimage 21:1585–1595
Poggesi A, Pracucci G, Chabriat H et al (2008) Urinary complaints in nondisabled elderly people with age-related white matter changes: the Leukoaraiosis and Disability (LADIS) Study. J Am Geriatr Soc 56:1638–1643
Pohjasvaara TI, Jokinen H, Ylikoski R et al (2007) White matter lesions are related to impaired instrumental activities of daily living poststroke. J Stroke Cerebrovasc Dis 16:251–258
Polvikoski TM, van Straaten EC, Barkhof F et al (2010) Frontal lobe white matter hyperintensities and neurofibrillary pathology in the oldest old. Neurology 75:2071–2078
Revesz T, Hawkins CP, du Boulay EP, Barnard RO, McDonald WI (1989) Pathological findings correlated with magnetic resonance imaging in subcortical arteriosclerotic encephalopathy (Binswanger’s disease). J Neurol Neurosurg Psychiatry 52:1337–1344
Ropele S, Seewann A, Gouw AA et al (2009) Quantitation of brain tissue changes associated with white matter hyperintensities by diffusion-weighted and magnetization transfer imaging: the LADIS (Leukoaraiosis and Disability in the Elderly) study. J Magn Reson Imaging 29:268–274
Rosano C, Sigurdsson S, Siggeirsdottir K et al (2010) Magnetization transfer imaging, white matter hyperintensities, brain atrophy and slower gait in older men and women. Neurobiol Aging 31:1197–1204
Sachdev P, Wen W, Chen X, Brodaty H (2007) Progression of white matter hyperintensities in elderly individuals over 3 years. Neurology 68:214–222
Scarpelli M, Salvolini U, Diamanti L, Montironi R, Chiaromoni L, Maricotti M (1994) MRI and pathological examination of post-mortem brains: the problem of white matter high signal areas. Neuroradiology 36:393–398
Scheltens P, Barkhof F, Leys D, Wolters E, Ravid R, Kamphorst W (1995) Histopathological correlates of white matter changes on MRI in Alzheimer’s disease and normal aging. Neurology 45:883–888
Schmidt R, Enzinger C, Ropele S, Schmidt H, Fazekas F (2003) Progression of cerebral white matter lesions: 6-year results of the Austrian Stroke Prevention Study. Lancet 361:2046–2048
Schmidt R, Hayn M, Fazekas F, Kapeller P, Esterbauer H (1996) Magnetic resonance imaging white matter hyperintensities in clinically normal elderly individuals. Correlations with plasma concentrations of naturally occurring antioxidants. Stroke 27:2043–2047
Schmidt R, Lechner H, Fazekas F et al (1994) Assessment of cerebrovascular risk profiles in healthy persons: definition of research goals and the Austrian Stroke Prevention study. Neuroepidemiology 13:308–313
Schmidt R, Petrovic K, Ropele S, Enzinger C, Fazekas F (2007) Progression of leukoaraiosis and cognition. Stroke 38:2619–2625
Schmidt R, Ropele S, Ferro J et al (2010) Diffusion-weighted imaging and cognition in the leukoaraiosis and disability in the elderly study. Stroke 41:e402–e408
Schmierer K, Altmann DR, Kassim N et al (2004) Progressive change in primary progressive multiple sclerosis normal-appearing white matter: a serial diffusion magnetic resonance imaging study. Mult Scler 10:182–187
Schmierer K, Scaravilli F, Altmann D, Barker G, Miller D (2004) Magnetization transfer ratio and myelin in postmortem multiple sclerosis brain. Ann Neurol 56:407–415
Schmierer K, Tozer DJ, Scaravilli F et al (2007) Quantitative magnetization transfer imaging in postmortem multiple sclerosis brain. J Magn Reson Imaging 26:41–51
Schmierer K, Wheeler-Kingshott CA, Boulby PA et al (2007) Diffusion tensor imaging of post mortem multiple sclerosis brain. Neuroimage 35:467–477
Shenkin SD, Bastin ME, Macgillivray TJ et al (2005) Cognitive correlates of cerebral white matter lesions and water diffusion tensor parameters in community-dwelling older people. Cerebrovasc Dis 20:310–318
Shepherd TM, Thelwall PE, Stanisz GJ, Blackband SJ (2009) Aldehyde fixative solutions alter the water relaxation and diffusion properties of nervous tissue. Magn Reson Med 62:26–34
Shereen A, Nemkul N, Yang D et al (2010) Ex vivo diffusion tensor imaging and neuropathological correlation in a murine model of hypoxia–ischemia-induced thrombotic stroke. J Cereb Blood Flow Metab. doi:10.1038/jcbfm.2010.212
Simpson JE, Fernando MS, Clark L et al (2007) White matter lesions in an unselected cohort of the elderly: astrocytic, microglial and oligodendrocyte precursor cell responses. Neuropathol Appl Neurobiol 33:410–419
Simpson JE, Ince PG, Higham CE et al (2007) Microglial activation in white matter lesions and nonlesional white matter of ageing brains. Neuropathol Appl Neurobiol 33:670–683
Simpson JE, Hosny O, Wharton SB et al (2009) Microarray RNA expression analysis of cerebral white matter lesions reveals changes in multiple functional pathways. Stroke 40:369–375
Smith CD, Snowdon D, Markesbery WR (2000) Periventricular white matter hyperintensities on MRI: correlation with neuropathologic findings. J Neuroimaging 10:13–16
Spilt A, Geeraedts T, de Craen AJ, Westendorp RG, Blauw GJ, van Buchem MA (2005) Age-related changes in normal-appearing brain tissue and white matter hyperintensities: more of the same or something else? AJNR Am J Neuroradiol 26:725–729
Spilt A, Goekoop R, Westendorp RG, Blauw GJ, de Craen AJ, van Buchem MA (2006) Not all age-related white matter hyperintensities are the same: a magnetization transfer imaging study. AJNR Am J Neuroradiol 27:1964–1968
Sze G, de Armond S, Brant-Zawadzki M, Davis R, Norman D, Newton T (1986) Foci of MRI signal (pseudo lesions) anterior to the frontal horns: histologic correlations of a normal finding. AJR Am J Roentgenol 147:331–337
Tanabe JL, Ezekiel F, Jagust WJ et al (1999) Magnetization transfer ratio of white matter hyperintensities in subcortical ischemic vascular dementia. AJNR Am J Neuroradiol 20:839–844
Taylor W, MacFall J, Provenzale J et al (2003) Serial MR imaging of volumes of hyperintense white matter lesions in elderly patients: correlation with vascular risk factors. Am J Roentgenol 181:571–576
Teodorczuk A, O’Brien JT, Firbank MJ et al (2007) White matter changes and late-life depressive symptoms: longitudinal study. Br J Psychiatry 191:212–217
Van der Flier W, van Straaten E, Barkhof F et al (2005) Small vessel disease and general cognitive function in non-disabled elderly: the LADIS study. Stroke 36:2116–2120
Van Dijk E, Prins N, Vermeer S et al (2004) Plasma amyloid beta, apolipoprotein E, lacunar infarcts, and white matter lesions. Ann Neurol 55:570–575
Van Dijk E, Prins N, Vermeer S, Koudstaal P, Breteler M (2002) Frequency of white matter lesions and silent lacunar infarcts. J Neurol Transm Suppl 25:25–39
Van Dijk E, Prins N, Vermeer S et al (2005) C-reactive protein and cerebral small vessel disease: the Rotterdam Scan Study. Circulation 112:900–905
Van Swieten J, van den Hout J, van Ketel B, Hijdra A, Wokke J, van Gijn J (1991) Periventricular lesions in the white matter on magnetic resonance imaging in the elderly: a morphometric correlation with arteriolosclerosis and dilated perivascular spaces. Brain 114:761–774
Viana-Baptista M, Bugalho P, Jordao C et al (2008) Cognitive function correlates with frontal white matter apparent diffusion coefficients in patients with leukoaraiosis. J Neurol 255:360–366
Wang L, Goldstein FC, Levey AI et al (2010) White matter hyperintensities and changes in white matter integrity in patients with Alzheimer’s disease. Neuroradiology. doi:10.1007/s00234-010-0806-2
Wen W, Sachdev PS, Chen X, Anstey K (2006) Gray matter reduction is correlated with white matter hyperintensity volume: a voxel-based morphometric study in a large epidemiological sample. Neuroimage 29:1031–1039
Young VG, Halliday GM, Kril JJ (2008) Neuropathologic correlates of white matter hyperintensities. Neurology 71:804–811
Acknowledgments
The immunohistochemical study on apolipoprotein E was supported by Grant 7776 of the Austrian National Bank (Jubilaeumsfonds) to Helena Schmidt.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schmidt, R., Schmidt, H., Haybaeck, J. et al. Heterogeneity in age-related white matter changes. Acta Neuropathol 122, 171–185 (2011). https://doi.org/10.1007/s00401-011-0851-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-011-0851-x