Abstract
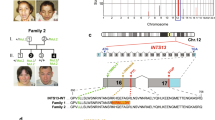
Congenital cataracts facial dysmorphism neuropathy (CCFDN) syndrome (OMIM 604168) is an autosomal recessive developmental disorder that occurs in an endogamous group of Vlax Roma (Gypsies; refs. 1–3). We previously localized the gene associated with CCFDN to 18qter, where a conserved haplotype suggested a single founder mutation4. In this study, we used recombination mapping to refine the gene position to a 155-kb critical interval. During haplotype analysis, we found that the non-transmitted chromosomes of some unaffected parents carried the conserved haplotype associated with the disease. Assuming such parents to be completely homozygous across the critical interval except with respect to the disease-causing mutation, we developed a new 'not quite identical by descent' (NQIBD) approach, which allowed us to identify the mutation causing the disease by sequencing DNA from a single unaffected homozygous parent. We show that CCFDN is caused by a single-nucleotide substitution in an antisense Alu element in intron 6 of CTDP1 (encoding the protein phosphatase FCP1, an essential component of the eukaryotic transcription machinery5,6), resulting in a rare mechanism of aberrant splicing and an Alu insertion in the processed mRNA. CCFDN thus joins the group of 'transcription syndromes'7 and is the first 'purely' transcriptional defect identified that affects polymerase II–mediated gene expression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tournev, I. et al. Congenital Cataracts Facial Dysmorphism Neuropathy (CCFDN) syndrome, a novel complex genetic disease in Balkan Gypsies: clinical and electrophysiological observations. Ann. Neurol. 45, 742–750 (1999).
Tournev, I., King, R., Muddle, J., Kalaydjieva, L. & Thomas, P.K. Peripheral nerve abnormalities in the congenital cataract facial dysmorphism neuropathy (CCFDN) syndrome. Acta Neuropathol. (Berlin) 98, 165–170 (1999).
Merlini, L. et al. Genetic identity of Marinesco-Sjogren/myoglobinuria and CCFDN syndromes. Neurology 58, 231–236 (2002).
Angelicheva, D., Tournev, I., Dye, D., Chandler, D., Thomas, P.K. & Kalaydjieva, L. Congenital cataracts facial dysmorphism neuropathy (CCFDN) syndrome: a novel developmental disorder in Gypsies maps to 18qter. Eur. J. Hum. Genet. 7, 560–566 (1999).
Chambers, R.S. & Dahmus, M.E. Purification and characterization of a phosphatase from HeLa cells which dephosphorylates the C-terminal domain of RNA polymerase II. J. Biol. Chem. 269, 26243–26248 (1994).
Archambault, J. et al. An essential component of a C-terminal domain phosphatase that interacts with transcription factor TFIIF in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 94, 14300–14305 (1997).
Vermeulen, W. et al. Three unusual repair deficiencies associated with transcription factor BTF2 (TFIIH): evidence for the existence of a transcription syndrome. Cold Spring Harb. Symp. Quant. Biol. 59, 317–329 (1994).
Müller-Felber, W. et al. Marinesco-Sjögren syndrome with rhabdomyolysis. A new subtype of the disease. Neuropediatrics 29, 97–101 (1998).
Kalaydjieva, L., Gresham, D. & Calafell, F. Genetic studies of the Roma (Gypsies): a review. BMC Med. Genet. 2, 5 (2001).
Kalaydjieva, L. et al. Patterns of inter- and intra-group genetic diversity in the Vlax Roma as revealed by Y chromosome and mitochondrial DNA lineages. Eur. J. Hum. Genet. 9, 97–104 (2001).
Gresham, D. et al. Origins and divergence of the Roma (Gypsies). Am. J. Hum. Genet. 69, 1314–1331 (2001).
Mitchell, G.A. et al. Splice-mediated insertion of an Alu sequence inactivates ornithine Δ-aminotransferase: A role for Alu elements in human mutation. Proc. Natl. Acad. Sci. USA 88, 815–819 (1991).
Lev-Maor, G., Sorek, R., Shomron, N. & Ast, G. The birth of an alternatively spliced exon: 3′ splice site selection in Alu exons. Science 300, 1288–1291 (2003).
Licciardo, P., Ruggiero, L., Lania, L. & Majello, B. Transcription activation by targeted recruitment of the RNA polymerase II CTD phosphatase FCP1. Nucleic Acids Res. 29, 3539–3545 (2001).
Maniatis, T. & Reed, R. An extensive network of coupling among gene expression machines. Nature 416, 499–506 (2002).
Chambers, R.S., Wang, B.Q., Burton, Z.F. & Dahmus, M.E. The activity of COOH-terminal domain phosphatase is regulated by a docking site on RNA polymerase II and by the general transcription factors IIF and IIB. J. Biol. Chem. 270, 14962–14969 (1995).
Cho, H. et al. A protein phosphatase functions to recycle RNA polymerase II. Genes Dev. 13, 1540–1552 (1999).
Cho, H., Kobor, M., Kim, M., Greenblatt, J. & Buratowski, S. Opposing effects of Ctk1 kinase and Fcp1 phosphatase at Ser 2 of the RNA polymerase II C-terminal domain. Genes & Dev. 15, 3319–3329 (2001).
Mandal, S.S., Cho, H., Kim, S., Cabane, K. & Reinberg, D. FCP1, a phosphatase specific for the heptapeptide repeat of the largest subunit of RNA polymerase II, stimulates transcription elongation. Mol. Cell. Biol. 22, 7543–7552 (2002).
Hengartner, C.J. et al. Temporal regulation of RNA polymerase II by Srb10 and Kin28 cyclin-dependent kinases. Mol. Cell 2, 43–53 (1998).
Washington, K. et al. Protein phosphatase-1 dephosphorylates the C-terminal domain of RNA polymerase 2. J. Biol. Chem. 277, 40442–40448 (2002).
Yeo, M., Lin, P.S., Dahmus, M.S. & Gill, G.N. A novel RNA polymerase II C-terminal domain phosphatase that preferentially dephosphorylates serine 5. J. Biol. Chem. 278, 26078–26085 (2003).
Lagier-Tourenne, C. et al. Homozygosity mapping of Marinesco-Sjögren syndrome to 5q31. Eur. J. Hum. Genet. (in the press).
Cottingham, R.W., Idury, R.M. & Shaffer, A.A. Faster sequential genetic linkage computations. Am. J. Hum. Genet. 53, 252–263 (1993).
Lathrop, G.M. & Lalouel, J.-M. Easy calculations of lod scores and genetic risks on small computers. Am. J. Hum. Genet. 36, 460–465 (1984).
Kruglyak, L., Daly, M.J. & Lander, E.S. Rapid multipoint linkage analysis of recessive traits in nuclear families, including homozygosity mapping. Am. J. Hum. Genet. 56, 519–527 (1995).
Reeve, J.P. & Rannala, B. DMLE+: Bayesian linkage disequilibrium gene mapping. Bioinformatics 18, 894–895 (2002).
Hunter, M. et al. The P28T mutation in the GALK1 gene accounts for galactokinase deficiency in Roma (Gypsy) patients across Europe. Pediatr. Res. 51, 602–606 (2002).
Nachman, M.W. & Crowell, S. Estimate of the mutation rate per nucleotide in humans. Genetics 156, 297–304 (2000).
Rogozin, I.B. & Pavlov, Y.I. Theoretical analysis of mutation hotspots and their DNA sequence specificity. Mutat. Res. 544, 65–85 (2003).
Acknowledgements
We thank affected individuals and their families for participating in this project; A. Corches, C. Lupu, M. Molnar, A. Kelemen, P. Seeman, V. Milic-Rasic for referring individuals with CCFDN; M. Delatycki, K. Jones, J. Colomer and T. Ishikawa for referring individuals with MSS; K. Sperling and J. Kunze for supporting this study; J. Reeve for help with the DMLE+ software; A. Usheva for discussions; N. Laing for critical comments on the manuscript; and D. Chandler and I. Martins for technical help. The study was funded by the National Health and Medical Research Council of Australia, The Wellcome Trust, the Australian Research Council, the Deutsche Forschungsgemeinschaft and partly by the German Ministry of Education and Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Varon, R., Gooding, R., Steglich, C. et al. Partial deficiency of the C-terminal-domain phosphatase of RNA polymerase II is associated with congenital cataracts facial dysmorphism neuropathy syndrome. Nat Genet 35, 185–189 (2003). https://doi.org/10.1038/ng1243
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1243
This article is cited by
-
CTDP1 regulates breast cancer survival and DNA repair through BRCT-specific interactions with FANCI
Cell Death Discovery (2019)
-
Deep intronic mutations and human disease
Human Genetics (2017)
-
Towards a functional pathology of hereditary neuropathies
Acta Neuropathologica (2017)
-
Breakpoints and deleted genes identification of ring chromosome 18 in a Chinese girl by whole-genome low-coverage sequencing: a case report study
BMC Medical Genetics (2016)
-
Histone acetylation and histone acetyltransferases show significant alterations in human abdominal aortic aneurysm
Clinical Epigenetics (2016)