Article Text
Abstract
Objective To establish the relative risks of in utero exposure to lamotrigine (LTG), sodium valproate (NaV) and carbamazepine (CBZ) monotherapy for neurodevelopment.
Design Observational cohort study.
Patients and methods The study group consisted of children in Northern Ireland aged 9–60 months born to mothers who had enrolled with the UK Epilepsy and Pregnancy Register. The control group consisted of children identified from the Child Health System database across Northern Ireland. Data were gathered on covariates recognised as influencing child development.
Main outcome measures Neurodevelopment assessed using either the Bayley Scales of Infant Development or the Griffiths Mental Development Scales.
Results 210 children underwent assessment by a single researcher blinded to antiepileptic drug exposure. 23 (39.6%) children exposed in utero to NaV, 10 (20.4%) exposed to CBZ and one (2.9%) exposed to LTG had evidence of mild or significant developmental delay, compared to two (4.5%) children in the control group. Multivariable analysis demonstrated that in utero exposure to NaV (OR 26.1, 95% CI 4.9 to 139; p<0.001) and to CBZ (OR 7.7, 95% CI 1.4 to 43.1; p<0.01) but not to LTG had a significant detrimental effect on neurodevelopment.
Conclusion In utero exposure to LTG did not have the detrimental effect on child development that was seen with NaV and with CBZ.
Statistics from Altmetric.com
Introduction
Infants born to women taking antiepileptic drugs (AEDs) in pregnancy are at increased risk of congenital malformations,1,–,6 particularly neural tube defects.7,–,9These concerns are, in part, responsible for the trend in AED prescribing from poly to monotherapy and from older to newer AEDs.10 The relatively recent introduction of such AEDs limits our awareness of their potential teratogenicity. There was a significant 10-fold increase in the prevalence of lamotrigine (LTG) prescribing in 12–18-year-old females between 1993 and 2006, with concurrent decreases in the prescribing of both sodium valproate (NaV) and carbamazepine (CBZ).10 The evidence base to validate this change in prescribing habit remains limited.11,–,13
Less is known about the longer term effects of AEDs in general. Recent studies have reported neurocognitive impairment following in utero exposure to specific AED monotherapies14,–,20 but, at present, there is insufficient evidence to advise women of the precise risks associated with individual AED exposure on their child's future development and cognition.21 22
What is already known on this topic
▶ In utero exposure to antiepileptic drugs (AEDs) can result in major and minor congenital malformations.
▶ More recently, additional educational needs and reduced verbal IQ have been reported following in utero exposure to sodium valproate (NaV).
▶ Over the last two decades there has been a change in prescribing habits from older to newer AEDs.
What this study adds
▶ This study is the first to compare neurodevelomental outcomes in a cohort exposed in utero to lamotrigine to a control group; no detrimental effect was identified.
▶ Neurocognitive impairment was identified in the cohort exposed to NaV and in that exposed to carbamazepine.
The aim of this study was to establish the relative risks of in utero exposure to specific AED monotherapies on neurodevelopment.
Methods
Study design
Study group
The study group consisted of infants and children in Northern Ireland born to mothers enrolled on the UK Epilepsy and Pregnancy Register. This register invites enrolment from women with epilepsy who are pregnant or are planning a pregnancy. Inclusion criteria were children born to mothers taking one of three AEDs (LTG, NaV or CBZ) as monotherapy throughout pregnancy. Exclusion criteria were women who started or stopped AED therapy in pregnancy or were taking any other prescribed medication other than folic acid or iron supplements. Children born before 35 weeks gestation and those older than 8 years of age were excluded.
Control group
Children were identified from the Child Health System database across Northern Ireland. Inclusion criteria were children born to healthy women not taking prescribed medication other than folic acid or iron supplements during pregnancy. Exclusion criteria included children born before 35 weeks gestation and those older than 8years of age.
Outcome measures
All children were evaluated by a single researcher blinded to AED exposure during the initial assessment stage. Each assessment consisted of a physical and neurodevelopmental examination using one of two age-standardised tools. The Bayley Scales of Infant Development23 was used in children aged 42 months and younger and the Griffiths Scale of Infant Development24 in children older than 42 months. Performance was classified as ‘significant delay’ (score ≥2 SD below the mean), ‘mild delay’ (≥1, <2 SD below the mean), ‘normal’ (mean ±1 SD) or ‘accelerated’ (≥1 SD above the mean).
Additional data collected on the child included information on birth details, general health, family history, parental educational attainment (maternal, paternal and combined) and parental employment status. Socioeconomic status (SES) was measured using the Townsend Index25 and the educational attainment of the father and mother was recorded using the 2001 Census classification.26
Investigator blinding ceased at this point so that additional pregnancy details could be explored including epilepsy status, seizure frequency, AED use and health behaviours (folic acid compliance, smoking habit and alcohol ingestion).
Data analysis
Cross-tabulations and χ2 tests were used to investigate unadjusted differences between groups when these were defined by categorical variables. Logistic regression was used to investigate delayed development as a function of group covariates. One-way analysis of variance was used to assess differences between groups when the dependent outcome to be examined was a continuously distributed variable. Both homogeneity of variance and normality of residuals were verified. We used SPSS v 17.0.
The study was approved by the Research Ethics Committee, Queen's University, Belfast in January 2002.
Results
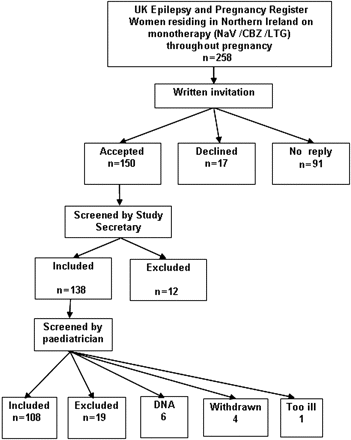
A written invitation to participate in the study was sent to 258 women enrolled on the UK Epilepsy and Pregnancy Register from 1996 to 2004. Recruitment was ongoing from May 2002 and assessment of offspring was carried out from August 2002 to February 2005. Replies were obtained from 167 mothers, with 150 consenting to participation (recruitment rate 58%) and 17 declining (figure 1). Of the 150 consenting mothers, 12 were excluded by the study secretary as they did not meet inclusion criteria (eg, AED stopped in pregnancy, medications other than AED in pregnancy, mother on polytherapy). Of the 138 mothers offered appointments, six failed to attend and five withdrew. The mothers of 333 control children were invited to participate; 83 replied, with 53 accepting and 30 declining.
Recruitment flow chart of mothers on the UK Epilepsy and Pregnancy Register. CBZ, carbamazepine; LTG, lamotrigine; NaV, sodium valproate.
Mothers
Of 127 women who attended appointments, 19 were excluded following assessment when blinding ceased and it was realised that they did not meet inclusion criteria (eg, non-compliance with AED), leaving 108 mothers on antiepileptic monotherapy throughout pregnancy who were included in the study; 45 (42%) women were on NaV, 37 (34%) on CBZ and 26 (24%) on LTG.
There was no significant difference in SES between the groups (p=0.093). Combined parental educational attainments identified a significantly lower attainment in the NaV group. Control mothers were significantly more likely to breast feed their infants than epileptic mothers. There was no significant difference in other factors examined (table 1).
Parental characteristics
Children
A total of 210 children born to 127 women were seen and assessed; however, 24 children of 19 mothers were excluded when AED non-compliance or comorbidity was disclosed by the mother at the end of the assessment. Fifty-eight children (31.2%) had been exposed throughout gestation to NaV, 49 (26.3%) to CBZ, 35 (18.8%) to LTG and 44 (23.7%) were controls (table 2). Sibships existed within each study group, with 12 in the NaV group (totalling 25 children), nine in the CBZ group (19 children), eight in the LTG group (17 children) and two in the control group (four children).
Characteristics of children
Children in the control group were significantly older at the time of assessment. There was no significant difference in growth measurements at birth (weight, length or head circumference). There was a trend for more preterm births in the exposed group compared to the control group.
Outcomes
Overall, 109 (58.6%) children were assessed using Bayley Scales of Infant Development and 77 (41.2%) using the Griffiths scales.
Numbers in both the accelerated and significantly delayed categories in all categories were small (figure 2) and these categories were dichotomised for analysis. Children in the normal and accelerated groups were amalgamated and children in the mild and significant delay groups were amalgamated. Of children born to mothers with epilepsy, 108 (76%) scored within the normal or accelerated ranges. However, children in the NaV and CBZ groups had an increased risk of delayed performance when compared to controls (table 3). The performance of children in the LTG group was comparable with that of the control group.
Neurodevelopmental outcomes in children exposed to antiepileptic drug monotherapies and in controls. CBZ, carbamazepine; LTG, lamotrigine; NaV, sodium valproate.
Unadjusted and adjusted summary statistics of covariates explored
Univariable analysis demonstrated that exposure in utero to either NaV or CBZ, age at assessment, birth weight, gender, SES, maternal educational attainment, seizure frequency in pregnancy (≥5 generalised tonic-clonic seizures) and duration of breast feeding had a significant association with neurodevelopmental outcome. Other covariates explored, including combined parental educational attainment, did not reach significance.
All factors reaching significance in univariable analysis were entered into a multivariable regression model, and if significance was retained they remained in the model, but if significance was lost they were removed. Variables were removed in a stepwise procedure. Table 3 reports the final model after a stepwise backwards procedure was invoked; we retained only the covariates which were significant at a level of 0.05. In utero exposure to either NaV or CBZ, but not to LTG, had a detrimental effect on neurodevelopment. The only confounding variables which persisted as significant adverse factors, but to a much lesser extent, were age, gender and SES.
We investigated the issue of greater concordance between siblings, but there was no tendency for there to be any greater agreement in classification of mental development within families than between children across families. Consequently, using all individuals as independent observants is justified in this study. We found that the component of variation at the level of cluster (family) was actually negative and hence there followed the conclusion that children from the same families were as variable as children from different families.
The predictive model created from this data for identifying those children at most risk of impaired neurodevelopment produces a receiver operating curve estimated at 0.86 (95% CI 0.80 to 0.92).
Discussion
The wellbeing of both mother and fetus must be considered in the choice of AED for women of childbearing age. The best drug for the mother is that which achieves optimum seizure control at the lowest dose with minimal side effects. Seizure control is also important for the fetus,27,–,29 but the potential for teratogenicity is equally important. Decisions are challenging when the drug with the lowest teratogenic profile provides less effective seizure control in the mother.
Our study found that children with a history of in utero exposure to either NaV or CBZ monotherapy were at increased risk of impaired neurodevelopment when covariates were considered in data analysis. There was no impairment in the neurodevelopment of children exposed in utero to LTG.
This study is one of the earliest to explore the effect of in utero exposure to LTG monotherapy on neurodevelopment. LTG has only been licensed for monotherapy use since 1995, constraining the numbers available for study inclusion and the age at follow-up. The results suggest that LTG does not adversely affect neurodevelopment, but longer term follow-up involving larger numbers of children is advised before LTG can be considered ‘safe’ for pregnant women. Our findings are consistent with those of a recent multicentre study.13 It is a relative strength of our study that exposed children were compared to a control group, thus avoiding like for like comparisons.
The present study reports on the largest cohort of children exposed to NaV monotherapy to date and supports previous reports of a detrimental effect on neurodevelopment.13,–,20 Previous studies examining the effect of in utero exposure to CBZ on neurodevelopment have mostly reported no significant impairment.15 16 30,–,35 Studies which have reported an adverse effect have tended to involve children presenting with dysmorphic facies and malformations, the ‘fetal CBZ syndrome’.17 36 The findings of our study suggest that isolated neurodevelopmental impairment is increased following in utero exposure to CBZ.
The identification of possible teratogenic effects of AEDs has largely been reliant on observational studies as it is neither ethical nor practical to perform randomised controlled trials on pregnant women. Our study recruited patients from the UK Epilepsy and Pregnancy Register, which enrols women with a broad range of epilepsy phenotypes from a wide variety of sources (including their family doctor, neurologist, obstetrician, midwife or self-referral) and is not confined to those with complex epilepsy syndromes. The register was established to facilitate the gathering of information on pregnancy outcomes, but is restricted in information collected on epilepsy phenotype and active documentation of seizure control and is reliant on maternal self-report, without biochemical confirmation, of AED and folic acid compliance. In addition, ethics approval for long term follow-up was not sought.
Strengths of our study were that all assessments were performed by a single researcher, reducing interobserver variability, and that the researcher was blinded to AED exposure. The recruitment rate (58%) is similar to other retrospective studies;17 19 higher rates in prospective studies have been reported.16 18 The number of children in the NaV group was higher than in similar studies. Two widely used30,–,36 age-standardised neurodevelopmental assessment tools accommodated both preschool and school age children. The inclusion of a control group allowed for potential cultural differences in children's abilities and the recognition that child performance has improved since the original standardisation.37
Limitations of this study, of necessity, are due to the relatively recent licensing of LTG for monotherapy, the limited information currently available from the UK Epilepsy and Pregnancy Register and the lack of consent for long term follow-up of the infants born to these mothers.
Women with comorbidities and babies born before 35 weeks gestation were excluded in order to strengthen methodology. Only three other studies reporting on AED exposure and neurodevelopment have actively sought information on potential confounding variables such as birth weight, gender, parental educational attainment, SES and the occurrence of generalised tonic-clonic seizures in pregnancy, all of which have the potential to negatively impact on neurodevelopmental outcomes in children.13 15 16
On multivariable regression analysis, male gender, older age of child and lower SES remained independent risk factors influencing neurodevelopment, but to a far lesser extent than exposure to NaV or CBZ. Children in the control group were significantly older, but when age was factored in to multivariable analysis, the effect of drug exposure became more significant. Of the other confounders explored, maternal educational attainment, seizures in pregnancy and AED dose produced trends in the directions anticipated but did not reach statistical significance. While it may be anticipated that prolonged maternal hypoxia would be detrimental to the developing fetal brain, to date, little is known about the effect of seizures in pregnancy on long term cognitive outcome. Studies reporting no detrimental effect are limited by small numbers of women with infrequent seizures.16 38 In our study, univariable analysis identified more than five generalised tonic-clonic seizures in pregnancy as having a significant detrimental effect on neurodevelopment, although this effect was lost on multivariable analysis.
Most of the children identified as having developmental delay in this study were in the ‘mildly delayed’ category. The implications of mild cognitive impairment for any child are uncertain, but early intervention has been shown to improve outcomes.39 While the school environment may help some children with mild impairment ‘catch up’ with peers, increasing academic demands may cause others to fall further behind. In light of the changes to child health surveillance,40 we suggest that children with a history of in utero exposure to AEDs should be identified and targeted for closer neurodevelopmental monitoring and early intervention as indicated.
In view of the growing evidence of adverse effects following in utero AED exposure, it is imperative to systematically evaluate newer drugs as they enter clinical practice. Larger multicentre studies with longer duration of follow-up, exploring the influence of potential confounding variables, are required. The roles of current pregnancy registers should be expanded, with standardisation of their methodologies and data collection, and inclusion of consent and ethics approval for follow-up. Future work should also examine the mechanisms by which AEDs adversely affect neurodevelopment and whether there are particularly vulnerable periods for the developing brain.
Acknowledgments
The authors would like to thank the UK Epilepsy and Pregnancy Register for their co-operation and support. The authors would also like to extend their gratitude to the parents and children who took part in this study.
References
Footnotes
-
Funding This study was funded by a fellowship from the Irish Institute of Neurology and Neurosurgery and a fellowship from the Royal Belfast Hospital for Sick Children. Neither source of funding had any involvement in the study design, data collection, analysis and interpretation, or in the writing and submission of this report.
-
Competing interests The UK Epilepsy and Pregnancy Register was made possible by a research grant from the Epilepsy Research Foundation and a number of unrestricted educational grants from pharmaceutical companies (Glaxo-Smith-Kline, Sanofi-Aventis, UCB-Pharma, Janssen-Cilag, Novartis, Pfizer and Eisai). Over the lifetime of the register, these grants have exceeded £10 000 from each company/grant awarding body. An internet-based website detailing the aims of the UK Epilepsy and Pregnancy Register was made possible by a grant from Glaxo-Smith-Kline and UCB-Pharma. JM has attended meetings with the support of various pharmaceutical companies, and has given lectures at the behest of pharmaceutical companies, for which he has received honoraria.
-
Ethics approval This study was conducted with the approval of the Research Ethics Committee, Queens University, Belfast (REC file number 322/01).
-
Provenance and peer review Not commissioned; externally peer reviewed.